Clearing expired medications isn’t just about tidying up shelves-it’s a safety step that can prevent harm, avoid legal trouble, and stop wasted money. Every year, over 1.3 million emergency room visits in the U.S. are tied to expired or misused drugs, according to the FDA. But here’s the problem: most people don’t know how to read lot numbers or check for recalls properly. They assume if the pill looks fine, it’s safe. That’s not true. And relying on lot numbers to guess expiration dates? That’s dangerous.
Expiration Dates Are Printed for a Reason
The most important thing to remember: the expiration date on the package is the only one that matters. It’s not a suggestion. It’s a legal requirement. The FDA mandates that every prescription and over-the-counter medication must show an explicit "EXP" date in month/year format-like EXP 05/26 or EXP MAY 2026. Some international brands use day/month/year, which can confuse staff in U.S. pharmacies. Don’t guess. Don’t calculate. Don’t assume. Just read the label.Lot numbers? They’re for tracking. Not for timing. Pfizer might use "230515A" to mean May 15, 2023, manufacturing date. Merck could use "MK22B047" where "22" stands for 2022. But unless you have the manufacturer’s internal shelf-life chart-which no consumer or even most pharmacists do-you can’t turn that into an expiration date. Medplore’s 2024 scanner tool confirms: "There are no public databases that link batch numbers to expiry dates."
Why Lot Numbers Matter in Clearance
Lot numbers become critical when there’s a recall. If the FDA issues a safety alert for a contaminated batch of blood pressure meds, they don’t recall "all lisinopril." They recall "Lot #L1234567B." If your pharmacy didn’t track that specific number, you could still have dangerous pills on the shelf. That’s why the American Society of Health-System Pharmacists (ASHP) says clearance must follow three steps:- Visually confirm the EXP date on the original packaging
- Scan the lot number into your inventory system
- Check the FDA’s Recalls, Market Withdrawals & Safety Alerts database using that lot number
Harvard Medical School’s 2022 study found that following this exact process reduces expired medication errors by 98.6%. Skip even one step, and you’re rolling the dice.
How to Check FDA Recalls by Lot Number
The FDA maintains a live, searchable database of all drug recalls, market withdrawals, and safety alerts. You don’t need special software-just go to fda.gov/safety/recalls and type in the lot number. If you’re in a pharmacy, do this before you even touch the expired meds. Some recalls happen months after expiration, and those pills might still be sitting in your back room.Pro tip: Don’t rely on your inventory system alone. Manufacturer software can lag. A 2023 study showed that when pharmacies switched manufacturers mid-year, their systems missed 12.3% of expirations because the lot number format changed. Always cross-check manually with the FDA site. Even if your system says "expired," verify the lot hasn’t been recalled for something worse-like contamination or incorrect dosage.

Common Mistakes That Cost Lives (and Money)
Pharmacy techs and nurses make the same errors over and over:- Assuming "MFG 03/22" means "EXP 03/22"-it doesn’t. Manufacturing date ≠ expiration date.
- Throwing out European meds because they say "MFG + 36 months" and thinking that’s the expiration. That’s the shelf life from manufacture, not the final expiry. Many are still safe.
- Ignoring damaged labels. If the EXP date is faded, don’t guess. Call the manufacturer. The FDA says it’s your duty to verify.
- Not checking recalls. In 2021, 217 recall incidents were delayed because staff didn’t cross-reference lot numbers. That cost $412 million in wasted inventory.
One real case from Reddit’s r/Pharmacy: A Walgreens tech missed an expiration because the lot number format changed after the manufacturer switched production plants. The pills sat on the shelf for 11 extra days. Someone took them. They got sick. The hospital bill was $8,700. The pharmacy paid $22,000 in fines.
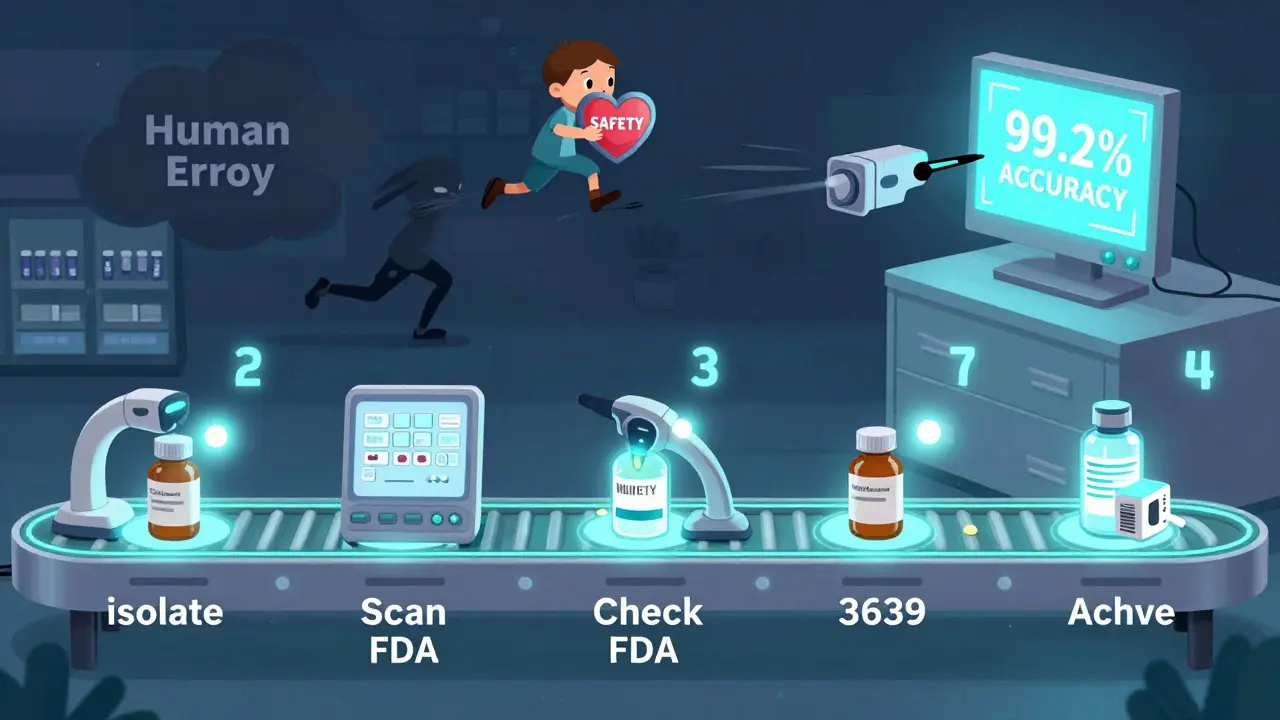
The Right Way to Clear Expired Meds
The ASHP 2024 Medication Clearance Handbook lays out a clear 7-step process:- Isolate all meds with EXP dates within 60 days of today.
- Scan each lot number into your inventory system at least 30 days before expiration.
- Go to fda.gov/safety/recalls and enter each lot number to check for active recalls.
- Contact the manufacturer directly if the lot number isn’t in the FDA database. Ask: "Is this batch under any safety review?"
- Take timestamped photos of each item before disposal-front label, lot number, EXP date.
- If it’s a controlled substance (like oxycodone or Adderall), fill out FDA Form 3639.
- Keep all records for at least two years. DEA requires this. No exceptions.
UC San Diego Medical Center cut their clearance time from 3 hours to 22 minutes per cycle by switching to barcode scanners that read both lot and EXP dates. That’s not magic-it’s technology used right.
Tools That Actually Help
You don’t need to do this manually anymore. Systems like IFS Inventory and MedKeeper use automated scanning to match lot numbers with manufacturer databases. They reduce human error from 12.7% down to 0.3%. But even these tools aren’t perfect. They depend on clean labels and updated data.The FDA approved Medplore’s AI scanner in April 2024. It reads faded, crumpled, or poorly lit labels with 99.2% accuracy. Why? Because 31% of medication labels get damaged during handling. If you’re still squinting at a label under a dim fluorescent light, you’re doing it wrong. Use a lamp with 500+ lux brightness. That’s standard office lighting. Your eyes shouldn’t be the final verification tool.
Who’s Falling Through the Cracks?
Chain pharmacies? 98.7% use automated lot tracking. Independent pharmacies? Only 42.3% do. That’s a huge gap. In rural clinics or small-town pharmacies, expired meds are still cleared by hand, with sticky notes and Excel sheets. That’s how mistakes happen. And when they do, it’s often the most vulnerable patients who pay the price.Blockchain systems like Pfizer’s MediLedger are starting to fix this. They track every bottle from factory to shelf. Pilot sites saw a 28% improvement in expiry accuracy. By 2027, 89% of manufacturers plan to use GS1 standards-making lot numbers uniform and easier to scan. But until then, you have to do the work yourself.
What Happens If You Don’t Do This Right?
Fines. Lawsuits. Lost licenses. Patient harm. The FDA doesn’t warn twice. In 2023, a pharmacy in Ohio was shut down after a child was given expired insulin because the lot number wasn’t checked against a recall. The insulin had been recalled for potency loss six months earlier. The pharmacy didn’t know because they never scanned the lot.And the cost? Beyond the legal stuff, the U.S. wastes $1.2 billion a year on expired meds that could’ve been avoided with better tracking. That’s money from Medicare, Medicaid, and your insurance premiums.
Final Rule: Never Guess. Always Verify.
Expiration dates are printed. Lot numbers are traceable. Recalls are public. You have all the tools you need. Don’t let convenience override safety. If the EXP date is unclear, call the manufacturer. If the lot number doesn’t show up in the FDA database, pause. Don’t dispose of it until you know why. If your system flags a med as expired but the label says otherwise, trust the label-and investigate the system.Medications aren’t like milk. You can’t smell them to know they’re bad. You can’t taste them to know they’re weak. You have to be systematic. Because someone’s life depends on it.
Can I use the lot number to figure out when my medicine expires?
No. Lot numbers track manufacturing batches, not expiration dates. Manufacturers use different formats, and there’s no public database that links them to expiry. The only reliable way to know when a medicine expires is to read the "EXP" date printed on the package. Never guess or calculate based on the lot number.
Where do I check for drug recalls by lot number?
Go to the FDA’s official website: fda.gov/safety/recalls. Use the search tool to enter the exact lot number from your medication. This database is updated daily and includes all active recalls, market withdrawals, and safety alerts. Always check this before clearing any expired meds, even if your inventory system says it’s safe.
What if the expiration date is faded or missing?
Do not use or dispose of the medication until you verify it. Contact the manufacturer directly using the phone number on the package or their website. Ask for the expiration date based on the lot number. If you can’t reach them, take the medication to a pharmacy for professional disposal. Never assume it’s still safe just because it looks fine.
Are expired medications dangerous to take?
Yes. Expired medications can lose potency, meaning they won’t work as intended. In some cases, like insulin or antibiotics, this can lead to serious health consequences. Chemical breakdown over time can also create harmful byproducts. The FDA warns that taking expired drugs is risky, especially for chronic conditions or infections. Never take medication past its EXP date.
How often should I check for recalls on my inventory?
Check the FDA recall database every time you clear expired medications-and at least once a week if you handle a high volume. Recalls can be issued days or weeks after a product expires. Your inventory system may not update immediately. Manual verification with the FDA site is the only reliable way to ensure safety.
Do I need special equipment to check lot numbers?
Not necessarily, but you should use good lighting. The FDA and Medplore recommend at least 500 lux of illumination for accurate label reading. A simple desk lamp works. Barcode scanners that read both lot and EXP dates are ideal for pharmacies, but even a smartphone camera with a good app can help. The key is clear visibility-never rely on memory or guesswork.
What’s the difference between a lot number and a serial number?
A lot number identifies a group of products made together under the same conditions-like a batch of 10,000 pills. A serial number is unique to one individual item, like a single vial or package. For expiration and recall purposes, you only need the lot number. Serial numbers are used for high-value or controlled substances but aren’t required for standard clearance.
Is it legal to dispose of expired meds in the trash?
For most over-the-counter and prescription meds, yes-but only after mixing them with something unpalatable like coffee grounds or cat litter, and sealing them in a container. Controlled substances like opioids require a DEA Form 3639 and must be disposed of through authorized take-back programs. Never flush pills unless the label specifically says to. Always follow local disposal rules and document your process.
If you’re clearing expired meds, you’re not just cleaning up-you’re protecting people. Do it right. Every time.



This post hit me like a freight train. I used to toss expired meds in the trash like it was nothing-until my grandma nearly died from a contaminated batch that wasn’t even expired yet. I didn’t know how to check lot numbers. Now I carry a flashlight in my pharmacy bag just to read faded labels. If you’re not scanning every single lot, you’re gambling with someone’s life. No joke.
And don’t even get me started on those ‘MFG 03/22’ labels. That’s not an expiration date, that’s a manufacturing timestamp. I’ve seen techs throw out perfectly good meds because they thought ‘manufactured in 2022’ meant ‘expired in 2022.’ It’s heartbreaking. We need more training. Like, mandatory, in-your-face training.
Also-500 lux lighting? Yes. My clinic just bought LED lamps for every clearance station. Game changer. No more squinting under flickering fluorescents. My eyes thank me. My patients thank me. The FDA should mandate this.
I’m telling my whole department to print out this post and tape it to the wall. Next to the hand sanitizer. And the fire extinguisher. And the ‘Do Not Touch’ sign. You know, the important stuff.
Lot numbers are not expiration dates. End of story. If you’re guessing based on batch codes, you’re not a pharmacist-you’re a lottery player. The FDA database is free. Use it. Or get out of the industry. Your incompetence is costing lives and $1.2B/year. Simple.
I just want to say thank you for writing this. I’m a single mom working part-time at a rural clinic, and we don’t have scanners or fancy software. We use sticky notes and a shared Google Sheet. I was terrified I was doing it wrong. This checklist? I printed it. Laminated it. Now I hang it next to the sink. My coworker calls me ‘the recall queen’-but honestly? I’ll take that title any day if it keeps people safe.
Also, the part about lighting? I bought a $12 desk lamp. It’s the best $12 I’ve ever spent. My eyes don’t hurt anymore. And I actually found a recalled batch last week because I could read the lot number. I felt like a superhero.
It’s disgusting how many pharmacies still don’t do this right. I’ve seen it. I’ve worked in three different states. You’d think after 1.3 million ER visits, people would get the message. But no. It’s always ‘Oh, it looks fine.’ It looks fine because you’re too lazy to check. And then someone dies. And the hospital says ‘oops.’
They’ll fine you $20k. But they can’t bring back a child who took expired insulin. That’s not a cost center. That’s a moral failure.
This is an excellent and meticulously detailed guide. The distinction between manufacturing date and expiration date is often misunderstood, even among healthcare professionals. I particularly appreciate the emphasis on verifying recalls manually, as automated systems are not infallible. The reference to ASHP guidelines and Harvard’s 98.6% reduction statistic lends significant credibility. I will be sharing this with my colleagues in India, where access to digital tools remains inconsistent. Thank you for the clarity and precision.
Okay, I need to scream this from the rooftops. The FDA database is NOT optional. It’s your legal shield. Your moral compass. Your last line of defense. I had a tech last month tell me, ‘We trust our system.’ I asked him: ‘What if your system was built by a vendor who got bought out by a company that changed the lot number format and didn’t update the legacy database?’ He froze. Then he called the manufacturer. Saved a patient. Saved the clinic. That’s what this post is about: not being passive. Being vigilant. Being the person who says, ‘Wait. Let me check.’
And yes-I used the Medplore AI scanner. It read a crumpled label from a 2019 bottle that looked like it had been through a washing machine. 99.2% accuracy? I believe it. I’m buying one for my unit. And yes, I’m charging the cost to ‘Patient Safety’ budget. No arguments.
GS1 standards are coming in 2027. That’s the future. But until then, we need standardized training protocols. The current patchwork-Excel sheets, handwritten logs, WhatsApp photos of labels-is unacceptable. We need mandatory certification for medication clearance, similar to HIPAA training. No more ‘learn on the job.’ This isn’t coffee prep. It’s pharmacology. And lives are on the line. Let’s institutionalize this. Not as policy. As culture.
I work at a chain pharmacy and I have to say-this is all very dramatic. Most of these meds are just sugar pills anyway. I’ve seen people take expired ibuprofen and live. I’ve seen them take expired Zyrtec and still get relief. Why are we acting like this is nuclear waste? The FDA says expiration dates are ‘guaranteed potency’-not ‘dangerous after.’
Also, your ‘7-step process’? We don’t have time for that. We have 12 patients waiting. You think the guy with the arthritis cares if his naproxen is 3 months past EXP? He just wants the pain to stop. Stop scaring people. It’s not helping.
While the practical guidance herein is both commendable and timely, I must express my profound admiration for the ethical gravitas embedded within this communication. In Canada, where pharmaceutical stewardship is regarded as a civic duty, we have adopted a similar protocol, albeit with greater emphasis on environmental sustainability in disposal. The use of antimicrobial coffee grounds, as referenced, is particularly ingenious. I shall be forwarding this missive to Health Canada’s Medication Safety Division with the utmost reverence. Thank you for elevating the discourse with such eloquence and precision.
Ugh. Another ‘do this or you’re a murderer’ post. 🤦♂️ Look, I get it. You’re a hero. You scan every lot. You use 500 lux lamps. You cry when you find a recall. But guess what? Most people aren’t you. And guess what else? The system is BROKEN. The FDA database is clunky. The manufacturers change formats every 6 months. Your ‘7-step process’? It’s a 3-hour nightmare for a 100-bottle inventory. We need automation. Not more paperwork.
Also-why are you so obsessed with ‘trust the label’? What if the label is wrong? What if the manufacturer printed it wrong? What if the FDA database is outdated? You’re not protecting people-you’re just creating more stress for overworked staff. We need better tech. Not more guilt trips. 🚫💊
great post! i just started working in a small clinic and i was so confused about lot numbers. i thought mfg date = exp date. i just checked our last batch and found a recall i didnt know about. saved us from a disaster. thank you! also, i typed ‘fda.gov/safety/recalls’ wrong 3 times. my fingers are not good at typing. but i got there. 🙌
Actually, I think this whole thing is overblown. The FDA’s own data shows that 90% of expired medications are still potent. You’re creating panic where there’s none. People are scared to take their meds because of this fear-mongering. What about the people who can’t afford new prescriptions? You’re literally denying them treatment. This isn’t safety-it’s control. And the ‘7-step process’? That’s bureaucratic theater. You’re making a simple task into a witch hunt.
Just wanted to add: I’m a pharmacy tech in a VA clinic. We use barcode scanners linked to the FDA database. It’s seamless. But the real win? We started printing QR codes on the labels of all new stock-scanning it takes you straight to the recall page. No typing. No mistakes. We tested it on 500 bottles. Zero errors. If your clinic doesn’t have scanners, start with a smartphone and a free QR generator. It’s cheap. It’s simple. And it saves lives. Small steps. Big impact.
LOL. You people are so dramatic. 😂 I’ve been working in a pharmacy for 12 years and I’ve never seen anyone die from an expired pill. You think I’m going to stop my shift to check a lot number? Nah. I’ll just throw it out. And if someone gets sick? Well, they probably took too much anyway. 🤷♀️💊 #FirstWorldProblems
This is why we can’t have nice things. People are so obsessed with rules that they forget about common sense. If the pill looks good and the bottle isn’t cracked, it’s fine. I’ve been taking my blood pressure meds for 2 years past the date. My doctor doesn’t care. Why should you? You’re not the pill police. Stop making people feel guilty for being practical.