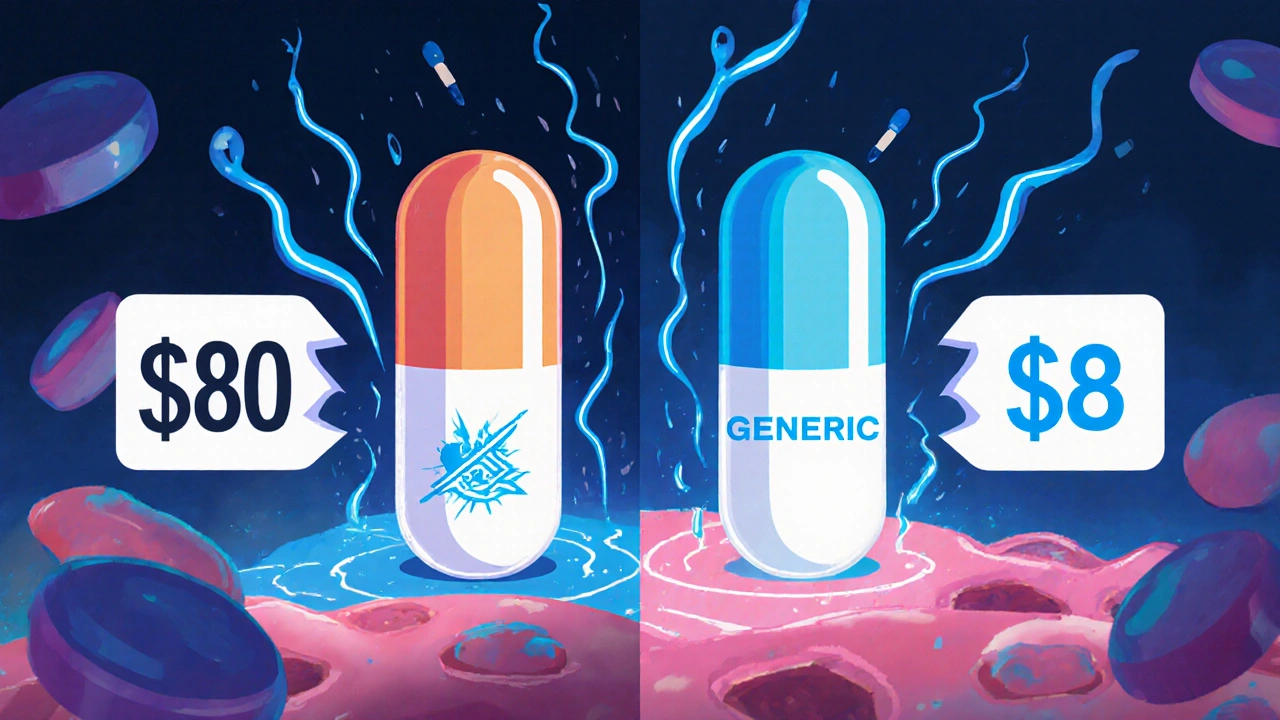
When you pick up a prescription, you might see two names on the bottle: one you recognize from TV ads, and another that looks completely different. One costs $8. The other costs $80. You might wonder: are generic drugs really the same? The short answer is yes-most of the time, they are. But there’s more to it than just price.
What Makes a Drug Generic?
A generic drug isn’t a copy. It’s not a knockoff. It’s an FDA-approved version of a brand-name drug that contains the exact same active ingredient, in the same strength, and works the same way in your body. The FDA requires generic manufacturers to prove their product delivers the same amount of medicine into your bloodstream at the same speed as the original. That’s called bioequivalence. The standard? Between 80% and 125% of the brand-name drug’s absorption rate. For drugs where tiny changes matter-like blood thinners or thyroid meds-that range is even tighter: 90% to 111%.Why Are Generic Drugs So Much Cheaper?
Brand-name drug companies spend years and hundreds of millions developing a new medicine. They run clinical trials, pay for marketing, and protect their invention with patents. Once that patent expires, other companies can make the same drug. They don’t need to repeat expensive trials. They just need to prove their version works the same way. That cuts their costs dramatically. The result? Generic drugs cost 80% to 85% less on average. In 2022, the average out-of-pocket cost for a generic prescription was $12.50. The same drug as a brand name? $68.30. That’s not a small difference-it’s life-changing for people managing chronic conditions like high blood pressure, diabetes, or cholesterol. Over a year, switching to generics can save hundreds or even thousands of dollars.What’s Different About Generic Drugs?
You’ll notice differences, but they’re not in how the drug works. Generics can look different-different color, shape, size, or even taste. That’s because U.S. trademark laws say a generic can’t look exactly like the brand-name version. The inactive ingredients (like fillers, dyes, or flavorings) might also vary. These don’t affect how the medicine works in your body. But if you’re allergic to a specific dye or have a sensitive stomach, those small changes might matter. For example, one person switching from brand-name Advair to its generic version reported zero change in asthma control-and saved $400 a month. Another person switching from brand Lamictal to generic lamotrigine noticed their seizures became harder to control. That’s rare, but it happens.When Generics Are the Clear Choice
Generic drugs shine for long-term, daily medications. If you’re taking something every day for years-like metformin for diabetes, lisinopril for blood pressure, or atorvastatin for cholesterol-the savings add up fast. In fact, 90% of all prescriptions filled in the U.S. are for generics. Yet they make up only 25% of total drug spending. That means brand-name drugs, which make up just 10% of prescriptions, are responsible for 75% of the cost. The FDA says it clearly: “All drugs, including brand-name and generic, must work well and are safe.” The American Medical Association agrees: doctors should prescribe generics when appropriate to cut costs without hurting outcomes.When You Might Want to Stick With Brand
There are exceptions. Some medications have a narrow therapeutic index-meaning even small changes in blood levels can cause problems. Warfarin (a blood thinner), levothyroxine (for thyroid), and certain seizure drugs like phenytoin fall into this category. While studies show no major difference between brand and generic versions of these drugs, some doctors and patients prefer sticking with one version for consistency. In 2019, a study of 38,000 people on levothyroxine found no clinical difference between brand and generic. But in real life, some patients report feeling off after switching. If you’ve been stable on a brand-name drug for years and your doctor sees no reason to change, staying put is fine. If you do switch, monitor how you feel. Tell your doctor if anything changes.What the Law Says About Substitution
In 49 out of 50 U.S. states, pharmacists are required to substitute a generic drug if one is available-unless the doctor writes “dispense as written” on the prescription. That means if your doctor prescribes Lipitor and there’s a generic version, the pharmacy will give you atorvastatin unless told otherwise. You don’t need to ask. It’s automatic. But you can ask. If you’re unsure, say something like: “Is there a generic available? Can we switch?” Your pharmacist can tell you what’s covered by insurance and how much you’ll save. Many people don’t realize they have the power to choose.What About Biosimilars?
Not all drugs can be copied easily. Biologics-drugs made from living cells, like insulin, rheumatoid arthritis treatments, and cancer therapies-are too complex to copy exactly. Instead, we have biosimilars. These are highly similar, but not identical, versions. They’re not called “generics,” but they serve the same purpose: lowering costs. The FDA approves them using strict standards, and they’re growing in number. By 2028, more than 450 brand-name drugs will lose patent protection, opening the door for even more generics and biosimilars.


Okay, but what about the time I switched to generic Lexapro and suddenly felt like a zombie who forgot how to feel joy?? I cried for three days. My therapist asked if I was okay. I said 'no' and handed her the pill bottle. That’s not science-that’s betrayal.
generic drugs are literally the same thing i swear on my grandmas boston cream pie the only difference is the color and the price and i saved like 500 bucks a year switching to generic metformin
Let’s be real here-the FDA’s 80-125% bioequivalence range is a joke. That’s a 45% swing in blood concentration. If you’re on warfarin or lithium, you’re basically playing Russian roulette with your liver. Pharma companies love generics because they’re a loophole to make more money off the same formula
I’ve been on generic lisinopril for 7 years. No side effects. No issues. My BP is better than it was on the brand. Save your money. Your future self will thank you.
I work in a clinic in rural Ohio. Every week, someone tells me they’re skipping meds because they can’t afford the brand. Then they switch to generic-and their eyes light up. It’s not magic. It’s justice. Let people live.
There’s a reason pharmacists are legally allowed to substitute generics-it’s backed by data. The FDA requires ANDAs (Abbreviated New Drug Applications) with rigorous bioequivalence testing. The inactive ingredients? They’re regulated too. If you’re allergic to FD&C Red No. 40, sure, check the label. But don’t confuse color with efficacy.
Canada doesn’t have this problem. We get generics at 90% off from day one. Americans are being scammed by corporate greed. Why do we let this happen?
I used to think generics were just cheap knockoffs, like buying a fake Gucci bag. But then I read the FDA guidelines, talked to my pharmacist, and realized-this isn’t about branding, it’s about biology. The molecule doesn’t care if it’s labeled ‘Lipitor’ or ‘atorvastatin.’ It just wants to lower your LDL. And honestly? That’s kind of beautiful.
Switched from Synthroid to generic levothyroxine and my TSH went from 2.1 to 6.8 in 6 weeks. Doctor said it was coincidence. I said no. Went back to brand. Back to normal. Don’t play with your thyroid. It’s not a game.
It’s important to distinguish between pharmacokinetic equivalence and clinical equivalence. While the former is statistically validated, the latter requires longitudinal patient-reported outcomes, which are often underreported in regulatory filings. The variability in excipients may induce subclinical inflammation in susceptible populations-particularly those with autoimmune comorbidities.
My mum’s been on the same generic statin since 2015. She’s 78. Still gardens. Still bakes pies. Still tells me off for not eating my vegetables. She says, ‘If it keeps me alive and doesn’t cost me a fortune, why would I complain?’ And honestly? She’s right.
My father is diabetic. He switched to generic metformin. His blood sugar improved. His monthly expense dropped from $140 to $12. He cried when he told me. Not because he was sad-because he finally felt like he could breathe.
Just switched to generic Lamictal last month. Zero issues. No seizures. No weird brain fog. Saved $300. I’m not a hero. I’m just someone who reads labels and trusts science 😊
It is a well-documented fact that generic medications meet the same standards as brand-name equivalents. The FDA’s bioequivalence requirements are stringent. Cost savings are not incidental-they are systemic and intentional. Patients should be empowered to choose generics without stigma or fear. Health equity begins with access.
And yet… my sister’s epilepsy specialist still refuses to let her switch from brand Lamictal. Even after 12 years of perfect control on generic. He says, ‘I’ve seen too many bad outcomes.’ So now she pays $600 a month… and we all just… accept it. Why?