Have you ever picked up your prescription and noticed the pill looks different? Maybe it’s a different color, shape, or even comes as a liquid instead of a tablet. You might wonder if it’s the same medicine-or if something’s been swapped out. This isn’t a mistake. It’s medication reformulation, and it’s happening more often than you think.
What Exactly Is a Drug Reformulation?
Medication reformulation means changing how a drug is made-without changing the active ingredient. The medicine still treats the same condition, but the way it’s delivered, absorbed, or even packaged has been updated. Think of it like upgrading your phone’s battery: the processor stays the same, but now it lasts longer and charges faster.
These changes can include:
- Switching from a tablet to a chewable, liquid, or patch form
- Changing how slowly or quickly the drug releases in your body (extended-release vs. immediate-release)
- Replacing one inactive ingredient (like a filler or dye) with another
- Improving how well the drug dissolves in your stomach to make it more effective
The active ingredient-the part that actually treats your illness-stays exactly the same. That’s what makes it a reformulation, not a new drug. If the chemical structure of the active ingredient changes, that’s a whole new drug, and it goes through a completely different approval process.
Why Do Companies Do This?
It’s not just about profit. While some reformulations are designed to extend patent life, many are driven by real patient needs.
Take a patient with arthritis who struggles to swallow large pills. A reformulated version that comes as a dissolvable tablet or a patch can make a huge difference in whether they take their medicine regularly. Or consider a child with epilepsy who can’t swallow capsules-switching to an oral suspension means they actually get the treatment they need.
Companies also reformulate to improve stability. Some drugs break down in heat or humidity. By changing the coating or packaging, they can make the medicine last longer on the shelf, especially in places without perfect storage conditions.
And then there’s the business side. Developing a brand-new drug can cost over $2.6 billion and take 10 to 15 years. Reformulation? That’s usually $50-100 million and 3 to 5 years. The approval success rate? Around 30%-three times higher than for brand-new drugs. For smaller companies or those working on rare diseases, this is often the only viable path forward.
How Is It Approved?
In the U.S., the FDA created a special pathway called 505(b)(2) in the 1980s to make reformulation easier. It lets companies use existing safety and effectiveness data from the original drug. They don’t have to start from scratch.
But here’s the catch: if the reformulation changes how the drug works in your body-like making it release slower or faster-you still need to prove it’s bioequivalent. That means your body absorbs it at the same rate and to the same extent as the original. This is tested in small clinical studies with healthy volunteers.
For example, if a drug used to be taken three times a day and is now reformulated to be taken once daily, the company must prove the once-daily version delivers the same total amount of medicine over 24 hours. Otherwise, you could be underdosed or overdosed.
Regulators don’t approve every change. If the reformulation doesn’t improve patient outcomes or safety, it can be rejected-even if it’s technically possible.
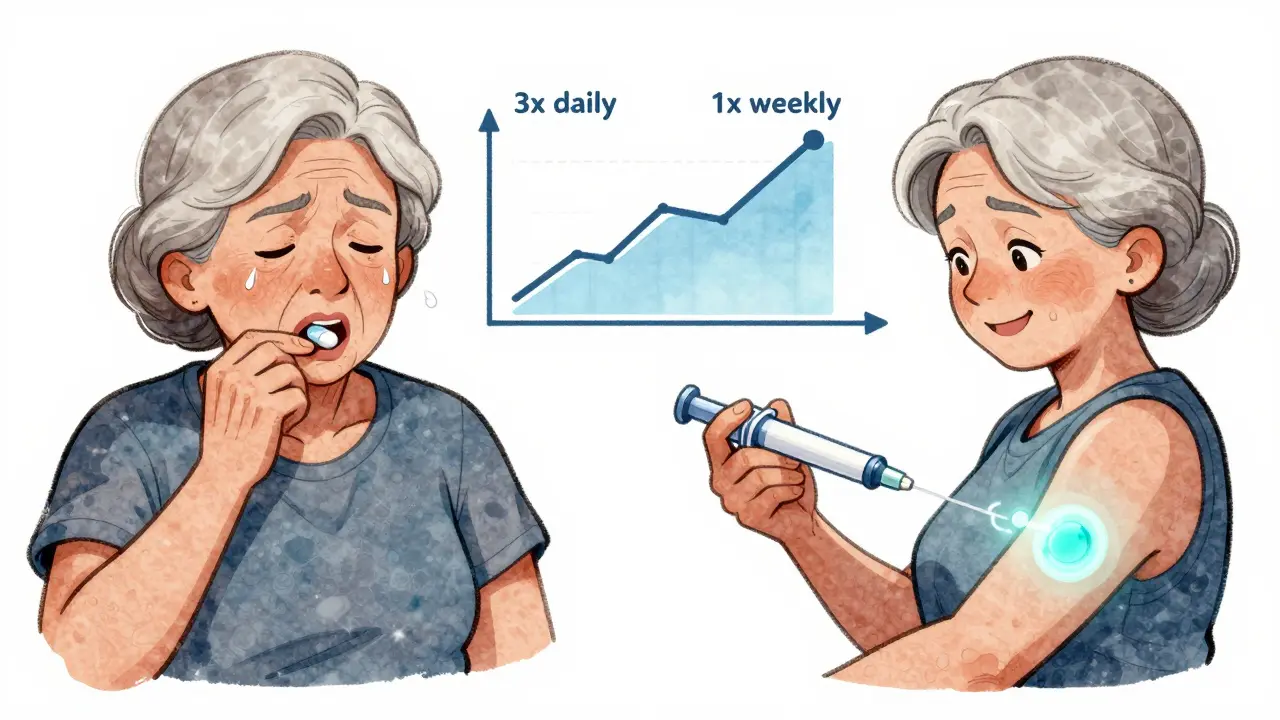
When Reformulation Helps Patients
Real-world examples show how powerful reformulation can be.
A mid-size pharmaceutical company in 2022 reformulated an orphan drug for a rare metabolic disorder. The original version required painful daily injections. The new version? A once-weekly subcutaneous injection with a smaller needle and easier-to-use pen device. Patient compliance jumped from 58% to 89% within six months.
Another case involved a blood pressure medication that caused stomach upset in 30% of users. By switching the tablet’s coating and removing a common filler, the reformulated version reduced gastrointestinal side effects by nearly half-without changing the drug’s effectiveness.
Even small changes matter. A reformulated asthma inhaler that’s easier to coordinate with breathing helped elderly patients use it correctly. Before, many were getting no medicine at all. After, hospital visits dropped.
These aren’t hypotheticals. They’re documented outcomes from real patients and real data.
The Dark Side: Evergreening and Hidden Costs
Not all reformulations are created equal. Some companies make tiny, meaningless changes just to reset the patent clock.
One well-known example: changing a pill’s color from blue to green and calling it a “new improved formula.” No change in dosage, release, or ingredients-just a new label. These practices are called “evergreening.” They don’t help patients. They just delay cheaper generics from entering the market.
Regulators are catching on. The FDA now requires reformulations to show “meaningful therapeutic advantage” if they want to block generics. But enforcement isn’t perfect. Some reformulations slip through with little clinical benefit.
And there’s another cost: confusion. Patients and pharmacists may not realize a reformulated drug isn’t interchangeable with the old version. A patient switching from one extended-release tablet to another that looks similar but releases differently could end up with too much or too little medicine in their system.

What Should You Do If Your Drug Changes?
If your medication suddenly looks different, don’t panic-but don’t assume it’s the same either.
- Check the label. The active ingredient name should be identical.
- Ask your pharmacist. They can tell you if it’s a reformulation and whether it’s interchangeable.
- Watch for side effects. If you feel different after switching-dizziness, nausea, lack of effect-contact your doctor. It could be the reformulation.
- Don’t stop taking it. Some patients stop because they think the new version is “fake.” That’s dangerous.
Pharmacists are trained to spot these changes. If they recommend switching back to the old version, listen. Sometimes the reformulation just doesn’t work for everyone.
What’s Next for Drug Reformulation?
The future of reformulation is getting smarter. New technologies are making it possible to tailor drug delivery in ways we couldn’t before.
Imagine a pill that releases medicine only when your body needs it-based on your body temperature or pH levels. Or patches that monitor your drug levels and adjust dosage automatically. These aren’t sci-fi. They’re in early testing right now.
Reformulation is also becoming a key tool for rare diseases. With only a few thousand patients worldwide, companies can’t afford the cost of developing a brand-new drug. But reformulating an existing one? That’s doable. In 2023, over 40% of new drug applications for orphan conditions were reformulations.
And with aging populations, more people need easier-to-take medicines. Reformulation is one of the fastest ways to make that happen.
It’s not a magic fix. But when done right, it’s one of the most underappreciated tools in modern medicine. It doesn’t make headlines like a cure for cancer-but it helps millions of people take their pills every day.
Are reformulated drugs less effective than the original?
No, not if they’re approved properly. Reformulated drugs must prove they’re bioequivalent to the original-meaning your body absorbs the same amount of the active ingredient. If the reformulation changes how the drug is released (like from immediate to extended), it’s still designed to deliver the same total dose over time. But if you notice new side effects or reduced effectiveness, talk to your doctor. Not all reformulations work the same for every person.
Can I switch back to the old version if I don’t like the new one?
Yes, but it depends on your insurance and pharmacy. Some insurers only cover the reformulated version because it’s cheaper. If you’re having problems, ask your doctor to write a medical exception. They can request the original version if the reformulation isn’t working for you. Pharmacists can also help you find alternatives.
Why does my pill look different even though it’s the same drug?
Manufacturers change the look of pills for many reasons: to avoid counterfeiting, to improve stability, to make it easier to swallow, or to comply with new regulations. Sometimes it’s just a new supplier using different dyes or fillers. The active ingredient is still the same. But if the change affects how the drug works-like its release rate-it should be clearly labeled. Always check with your pharmacist if you’re unsure.
Do generic drugs count as reformulations?
No. Generics are copies of the original drug, made after the patent expires. Reformulations are changes made by the original manufacturer (or a licensee) while the patent is still active. A generic version of a reformulated drug is still considered a generic-it’s just a copy of the updated version. The key difference is who made the change and when.
Are reformulated drugs more expensive?
Often, yes-especially right after launch. Companies price reformulations higher to recoup development costs and maintain profits before generics arrive. But over time, if multiple companies reformulate the same drug, prices can drop. Some reformulations are covered by insurance without extra cost, especially if they improve adherence. Always compare prices and ask about generic alternatives.
Final Thoughts
Medication reformulation isn’t just corporate strategy-it’s patient care in disguise. The best reformulations don’t just extend patents. They make pills easier to take, reduce side effects, and help people stick to their treatment. But they’re not perfect. Some changes are meaningless. Some cause unintended problems.
The key is awareness. Know that your drug can change. Ask questions. Don’t assume a new-looking pill is the same. And if something feels off, speak up. Your health matters more than a company’s patent timeline.


This is why I hate big pharma. One day my pill is blue, next day it's green and suddenly I'm dizzy as hell. No warning, no explanation. Just 'trust us' while they reset their patent clock. 🤦♀️
As a pharmacist, I see this all the time. Patients panic when the pill changes color, but 90% of the time it’s just a different filler. Always check the active ingredient. If it’s the same, it’s probably fine. But if you feel weird? Come back. We’ll check the bioequivalency data.
Oh my god, I had this exact thing happen with my antidepressant. I switched from the white oval to this tiny blue round one and I swear I felt like I was falling into a black hole for two weeks. My therapist thought I was relapsing. Turns out the new version released too fast and my serotonin spiked then crashed. I had to fight my insurance for three months to get the old one back. They said the new one was 'more cost-effective.' More cost-effective for them, not for me. I’m still mad. I cried in the pharmacy aisle. I just wanted to feel normal again. And now they’re pushing this new patch version? No. Just no. I’m not a lab rat. 🫠
Reformulations aren't inherently bad. But the FDA's 505(b)(2) pathway is too lenient. Too many 'me-too' versions get approved with minimal data. If you change the release profile, you need real clinical outcomes, not just bioequivalence in 20 healthy volunteers. That's not medicine. That's math with a placebo effect.
Let me get this straight: we’re celebrating drug companies changing pill colors as 'patient care'? 😂 When the CEO gets a $50M bonus for turning a $0.10 tablet into a $5 patch with a 'new delivery system' that does absolutely nothing except delay generics, that’s not innovation. That’s corporate theft wrapped in a lab coat. And you call this 'underappreciated'? No. It’s under-EXPOSED.
Y’all need to stop freaking out and start advocating. Reformulation isn’t the enemy - lack of transparency is. If your pill changes, ask your doc to write a letter to the pharmacy: 'Patient experiences adverse effects with reformulated version. Please restore original formulation.' It works. I’ve done it 3 times. Also, if you’re on a rare disease med, thank a reformulator. That’s how my cousin got her seizure med in liquid form. She’s 8. She can’t swallow pills. The patch? She hates it. But the liquid? She drinks it like juice. That’s magic. 🌟
India’s generic industry saved millions. But when American companies repackage the same damn drug and charge 10x, it’s criminal. I saw a man in Delhi crying because his insulin cost $3. His son in Chicago pays $300 for the same vial. Reformulation? More like exploitation. You think your 'improved' coating matters when people are choosing between food and medicine? Wake up.
What is a drug, really? Is it the molecule? Or is it the ritual? The shape, the color, the way you hold it before swallowing - that’s part of the treatment. When you change the pill, you change the patient’s relationship to healing. That’s why some people feel worse even when the science says it’s identical. The mind doesn’t care about bioequivalence. It cares about continuity. We treat bodies like machines, but we’re not machines. We’re stories. And stories need familiar symbols.
Just a heads up - if your new pill tastes weird, it’s probably the coating. I had a statin that went from chalky to minty. Thought I was hallucinating. Turns out they swapped the flavoring. No effect on the drug, just your tongue. But if it’s making you gag? Tell your pharmacist. They can switch you to a capsule or liquid. Easy fix. Don’t suffer in silence. 🤢
Why are we even talking about this? America’s healthcare system is broken. We’re arguing over pill colors while people die because they can’t afford insulin. Reformulation? It’s a distraction. Fix the system first. Then we’ll talk about whether your antidepressant is blue or green.
Just wanted to say - I love how this post didn’t just say 'trust the science.' It acknowledged that people feel things. And that matters. 💛 I switched to a reformulated thyroid med and I swear I had brain fog for weeks. My doctor said 'it’s bioequivalent.' I said 'but I feel like a zombie.' He listened. We switched back. Science is important. But so is your lived experience. Never let them gaslight you out of your own body.
Big Pharma is a scam. They make a drug, wait for the patent to expire, then 'reformulate' it into a new version that costs 10x more. Then they sue generics for 'copying' the new version. It’s like changing the label on a Coke can and calling it 'Coke Plus.' Same sugar. Same water. Same lies. I’m not taking it anymore. I’m going back to herbal tea. At least I know what’s in it.
Look, I get it. Some reformulations help. But most of the time, it’s just marketing. If you’re not a doctor or a pharmacist, you’re not supposed to care. Just take the pill. Don’t question it. Don’t read the label. Don’t compare. Just swallow. Your job is to be compliant. Not to be a detective. The system works better if you don’t ask questions.
I’ve worked in UK pharmacy for 25 years. We don’t have this chaos. If a drug is reformulated, the NHS flags it in the system. Pharmacists get notified. Patients get a leaflet. No surprises. Here, it’s like a game of musical pills. One day you’re fine, next day you’re dizzy, and nobody takes responsibility. It’s not innovation. It’s negligence dressed up as progress.