When you take a new prescription, you might assume your doctor and the FDA have already checked every possible way it could react with your other meds, foods, or health conditions. But here’s the truth: many dangerous drug interactions are only found after millions of people have already been using the drug. This isn’t a flaw in the system-it’s how the system actually works. And understanding it could save your life.
Why Clinical Trials Miss So Much
Before a drug hits shelves, it goes through clinical trials. These studies are strict, controlled, and usually involve between 1,000 and 5,000 people. They’re designed to answer one main question: Does this drug work for its intended use? But they’re not built to find every risk. Think about it. Most trials exclude older adults, pregnant women, people with multiple chronic diseases, or those taking five or more medications. Yet in the real world, the average American over 65 takes four prescriptions. A trial might test a new blood pressure drug with healthy 50-year-olds for six months. But what happens when a 72-year-old with kidney disease, diabetes, and atrial fibrillation takes it for ten years-along with statins, antibiotics, and grapefruit juice every morning? That’s where things go wrong. A 2020 study found that pre-market trials catch only about half of common side effects. Post-market surveillance catches 70-80% of the serious ones. Why? Because real life is messy. And the real world has millions of patients, not thousands.How Dangerous Interactions Hide Until It’s Too Late
Some of the most shocking drug safety stories came out long after approval. Take terfenadine (Seldane), an antihistamine once sold over the counter. It was fine for most people. But when taken with certain antifungals like ketoconazole, it caused fatal heart rhythm disruptions. The interaction was missed in trials because those combinations weren’t tested. Once it hit the market, dozens of deaths were reported. The drug was pulled in 1998. Another example: simvastatin (Zocor) and fluconazole (Diflucan). Fluconazole blocks the liver enzyme CYP3A4, which breaks down simvastatin. Without that enzyme, simvastatin builds up in the blood-sometimes by 3 to 10 times. That can cause rhabdomyolysis, a condition where muscle tissue breaks down and floods the kidneys with toxic proteins. The FDA’s own data from 2015-2020 showed nearly 3,000 reports of this exact interaction. One user on Reddit wrote: “My doctor didn’t warn me. I ended up in the ER with kidney damage.” Even something as simple as grapefruit juice can be deadly. It blocks the same enzyme. One study found it can raise atorvastatin (Lipitor) levels by up to 15 times. That’s not a minor side effect-it’s a medical emergency waiting to happen.What Happens After the Drug Is Approved
Once a drug is on the market, surveillance kicks in. In the U.S., the FDA runs FAERS (FDA Adverse Event Reporting System). It’s not perfect. Only about 5-10% of actual adverse events get reported. Doctors and patients don’t always connect a reaction to a drug. But when enough people report the same problem-like sudden muscle pain, unexplained bleeding, or heart palpitations-a pattern emerges. The FDA’s Sentinel Initiative now monitors over 300 million patient records from hospitals, insurers, and pharmacies. It uses AI to spot unusual spikes in side effects. In Europe, EudraVigilance does the same with 2.1 million annual reports. These systems caught the link between benfluorex (Mediator) and heart valve damage after 5 million people had taken it for 30 years. The drug was withdrawn in 2009. But detection takes time. The extended-release painkiller Exalgo was found to cause dangerous “dose dumping” when taken with alcohol-only after 18 months on the market. By then, people had already overdosed.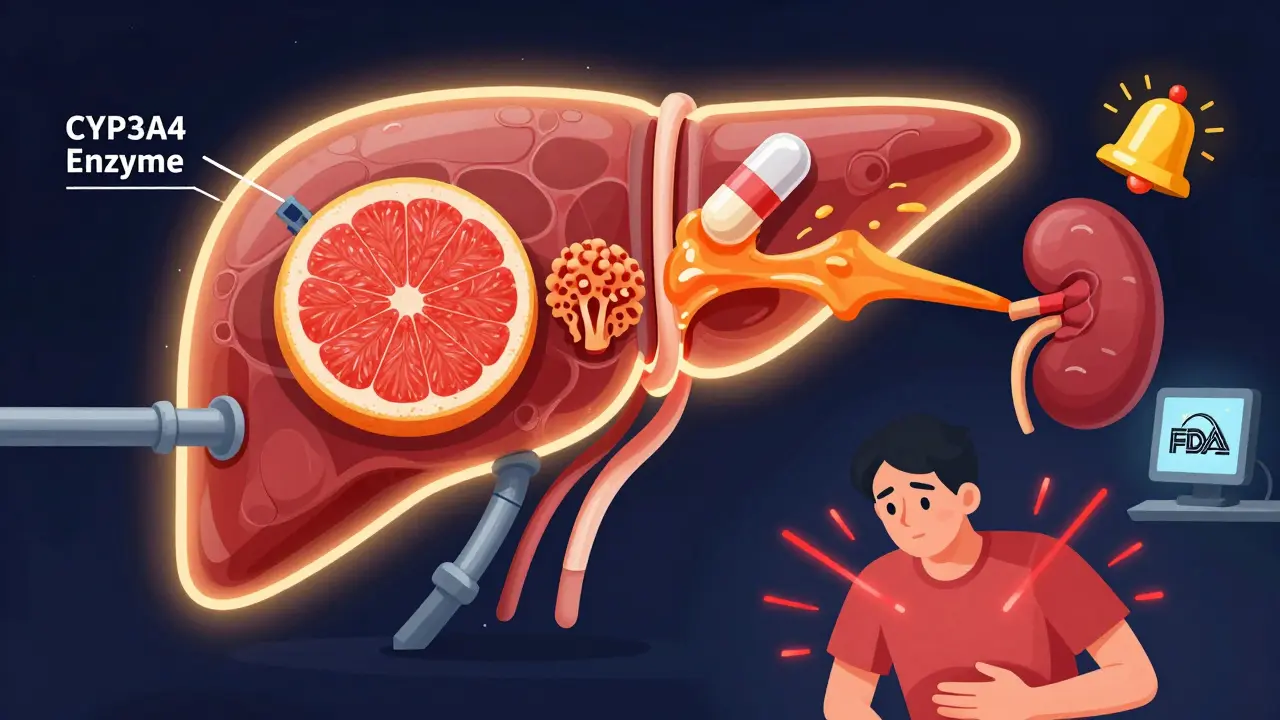
The Real-World Cost of Missing These Warnings
This isn’t just about individual harm. It’s expensive. The Institute of Medicine estimated in 2006 that drug interactions cost the U.S. healthcare system over $1 billion a year. That’s mostly from hospitalizations, ER visits, and long-term care. A 2022 analysis found that 15-20% of hospital admissions in the U.S. involve adverse drug events, and a large chunk of those are from interactions. One woman in her 70s started taking apixaban (Eliquis) for atrial fibrillation. She was also taking St. John’s Wort, a popular herbal supplement for depression. The interaction increased her bleeding risk. She ended up with a brain hemorrhage. Her case was reported to the FDA in 2022. The warning wasn’t clear on the label. Pharmaceutical companies now spend billions on post-market safety. The global pharmacovigilance market grew from $5.8 billion in 2020 to $7.3 billion in 2022. Nearly half of all new drugs now require post-approval studies focused on drug interactions. The FDA mandates these for 45.7% of new drugs. In the EU, it’s required by law.What You Can Do to Protect Yourself
You can’t rely on your doctor to catch every interaction. They’re human. They’re overwhelmed. A 2022 study found that even pharmacists miss up to 40% of high-risk combinations if they’re not using digital tools. Here’s what works:- Use a drug interaction checker-like GoodRx, Medscape, or your pharmacy’s app. One user wrote: “The warning prevented me from taking ciprofloxacin with my blood pressure meds. My pharmacist said it could’ve caused QT prolongation-could’ve killed me.”
- Always list every medication-including supplements, OTC painkillers, herbal products, and even alcohol. St. John’s Wort, garlic pills, and ginkgo biloba are notorious for interactions.
- Ask your pharmacist when you pick up a new script. They’re trained to spot these issues. And they have access to real-time databases your doctor doesn’t always use.
- Know your body. If you start feeling unexplained muscle pain, dizziness, bruising, or irregular heartbeat after starting a new drug-call your provider. Don’t wait.
The Future: AI, Genes, and Blockchain
The system is getting smarter. In January 2023, the FDA approved the first AI platform that can review 10,000 adverse event reports a day with 92.7% accuracy. The European Medicines Agency cut signal detection time from 18 months to 45 days using machine learning. Soon, your genes may play a role. The NIH’s Pharmacogenomics Research Network-2 is analyzing how DNA affects drug metabolism. Some people naturally break down statins slowly. Others process blood thinners too fast. Genetic testing could one day tell you if a drug is risky for you before you even take it. And blockchain? Companies are testing it to track adverse events securely and anonymously. The goal: fix underreporting. Right now, 90-95% of events go unreported. That’s not just a gap-it’s a danger zone.Bottom Line
Drug interactions discovered after approval aren’t failures. They’re proof that the system is working-just slowly. The same drugs that save lives can also kill if we don’t watch for hidden risks. The best protection isn’t regulation. It’s awareness. It’s asking questions. It’s using tools. It’s knowing that what’s on the label today might not be the full story tomorrow.Your health is your responsibility. Don’t assume safety. Check it.
What does it mean when a drug has a black box warning?
A black box warning is the strongest safety alert the FDA can require. It appears on a drug’s label and highlights serious or life-threatening risks-often discovered after the drug has been on the market. About 20% of new drugs get this warning post-approval, usually because of dangerous interactions, organ damage, or increased risk of death. Examples include statins with certain antifungals, or blood thinners with herbal supplements.
Why aren’t all drug interactions found before approval?
Clinical trials are too small, too short, and too controlled. They usually test drugs on healthy volunteers or patients with one condition. Real-world patients often have multiple illnesses, take five or more medications, are elderly, or have liver/kidney problems-all of which change how drugs behave. Interactions that only show up after years of use, or in rare combinations, simply don’t show up in trials.
Can grapefruit juice really make my medication dangerous?
Yes. Grapefruit juice blocks the CYP3A4 enzyme in the gut and liver, which breaks down many common drugs. For some medications, like atorvastatin (Lipitor), it can raise blood levels by up to 15 times. This can lead to severe muscle damage, kidney failure, or even death. The same goes for certain blood pressure meds, anti-anxiety drugs, and immunosuppressants. Always check if your drug has a grapefruit warning.
How do I know if my symptoms are from a drug interaction?
If you start new symptoms-like unexplained muscle pain, dizziness, confusion, unusual bleeding, or irregular heartbeat-within days or weeks of starting a new drug or supplement, it could be an interaction. Write down what you’re taking, when you started it, and when symptoms began. Bring this to your doctor or pharmacist. The Naranjo Algorithm, used by pharmacovigilance experts, helps assess if a drug caused the reaction based on timing, improvement after stopping, and other possible causes.
Are herbal supplements safer than prescription drugs?
No. Many people assume supplements are harmless, but that’s not true. St. John’s Wort, for example, can reduce the effectiveness of birth control, antidepressants, and blood thinners. Garlic and ginkgo can increase bleeding risk with anticoagulants. A 2022 FDA report described a life-threatening bleed from combining apixaban (Eliquis) with St. John’s Wort. Always tell your provider about everything you take-prescription, OTC, or herbal.
What’s the best way to check for drug interactions?
Use a trusted, up-to-date drug interaction checker like GoodRx, Medscape, or your pharmacy’s app. Ask your pharmacist to run a check every time you get a new prescription. If you take more than three medications, consider scheduling a medication review once a year. Electronic health records now connect to the FDA’s Drug Interaction API, which processes over 2.5 million queries daily-so your doctor’s system may already be flagging risks. But don’t rely on it alone.


